 Graves’ Hyperthyroidism
Graves’ Hyperthyroidism
Remission with Iodine
Case Report
by Jeffrey Dach MD
Carol is a 56 year old real estate agent who noticed a feeling of nervousness, warmth and rapid heart rate which worsened over a few days. Carol called a friend who drove her to the Emergency Room where the doctors gave her medications to slow the rapid rate (propranolol, a beta blocker). Lab testing confirmed she had Graves’ hyperthyroidism. Carol was sent home with an appointment to see an endocrinologist a week later.
The Endocrinologist
The endocrinologist saw Carol and ran a thyroid lab panel showing a TSH of .001, which is very low. In addition, the other lab tests showed a FreeT3 of 1200 and a FreeT4 of 4.4, both markedly elevated. Her Thyroid Stimulating Immune-globulin (TSI) test was also very elevated, indicating Graves’ Hyperthyroidism. This is an autoimmune disease in which antibodies attack and stimulate the TSH receptor of the thyroid gland causing high thyroid function. Carol was started on a thyroid blocking drug, methimazole 30 mg daily.
Carol Goes to a Health Resort
Unhappy with conventional treatment, Carol traveled to health resort ranch in Arizona specializing in organic raw vegetarian meals and fresh vegetable juices. She went to daily yoga classes, meditation and sauna treatments. The doctor at the Health Resort started Carol on a vitamin supplement program for her thyroid condition which included a potassium iodide capsule containing 65 mg of iodide.
Carol Starts to Feel Better !!
At the Health Ranch, Carol started feeling much better, almost normal, and her repeat her lab panel showed the TSH had gone back up to the normal range of 3.2. The other thyroid labs, the FreeT3 and FreeT4 had also normalized. However, the TSI remained quite elevated with little change.
Carol returned home and visited the endocrinology office. Her endocrinologist reviewed the labs, and then stopped the methimazole blocking drug. He said it was no longer needed. However, the Graves’ antibodies, (the TSI thyroid stimulating antibodies), were still very elevated, so the endocrinologist recommended a subtotal hyperthyroidectomy, a surgical procedure to remove the thyroid gland. Carol was unhappy with this recommendation. She was not keen on having thyroid surgery, and came to see me in the office to get a second opinion.
Coming for a Second Opinion
This case illustrates the beneficial effect of Iodine on Graves’ Disease, showing complete remission with a 65 mg potassium iodine tablet given at a health resort as part of a vitamin program.(1)
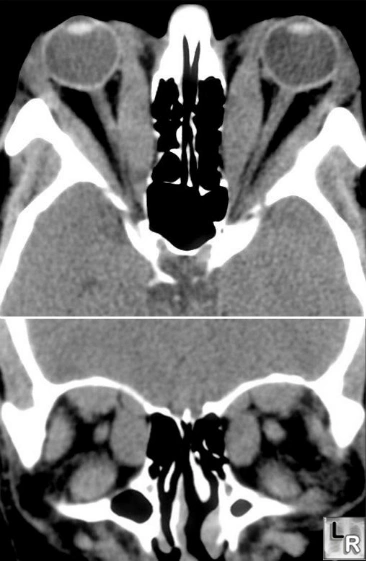 Left
Image shows CAT scan of exopthalmos caused by hypertrophied
extra-ocular muscles in Graves Disease. If severe, the optic nerve can
be compromised at the orbital apex resulting in loss of vision.
Antigens in the extraocular muscles and orbital fat may be attacked by
the autoimmune disease process.
Left
Image shows CAT scan of exopthalmos caused by hypertrophied
extra-ocular muscles in Graves Disease. If severe, the optic nerve can
be compromised at the orbital apex resulting in loss of vision.
Antigens in the extraocular muscles and orbital fat may be attacked by
the autoimmune disease process.
The History of Iodine Use for Hyperthyroidism- Exopthalmos Goiter
1811- Discovery of Iodine by Courtois
In 1811, Bernard Courtois, a French chemist accidentally discovered a purple substance which he named, Iodine.(1)
1863 – Dr. Trousseau Accidently Discovers Iodine Cures Graves Disease.
In 1863, Trousseau was called to visit a sick woman with tachycardia caused by Graves’ Hyperthyroidism.(2) Dr. Trouseau intended to write a prescription for tincture of Digitalis to slow the heart rate, but instead wrote for tincture of Iodine by mistake,
Upon initial examination the woman’s heart rate was 140 to 150 times per minute. When Trousseau returned the next day, the lady’s heart rate had slowed to normal. It was then he realized his mistake and discovered the patient actually took 75-100 mg of Iodine over night. He cancelled the Iodine and again prescribed tincture of Digitalis.
The next day, Trousseau again examined the patient and found the pulse had again gone up to 150 beats per minute. Trousseau realized the Iodine induced a beneficial slowing of the heart rate, and remission of hyperthyroid symptoms. Trousseau then returned to the use of iodine, placing the patient back on her original iodine prescription. (2)

Above
image : microscopic view of histology of normal thyroid gland showing
thin layer of thyrocytes outlining the rounded lakes of colloid. These
are called follicles.
Iodine – How does it work in Graves ?
It has been more than 150 years since Trousseau’s accidental discovery of the beneficial effect on Graves’ Disease patients. The question you might ask is: How does it work? What is the effect of Iodine on thyroid cell physiology? A basic science study by Corvilain in 1988 explains that Iodide inhibits hydrogen peroxide generation in the thyroid follicle, an important step in the organification of iodine to the thyroglobulin molecule.(3) Thus by inhibiting organic binding, Iodine inhibits formation and release of thyroid hormone by the thyroid gland, and thyroid levels decline promptly. This explains remission in Graves Hyperthyroidism.(19-21)

Left Image: Iodine in flask with purple color.
In 1920′s, Drs Plummer, Starr, Lahey and Thompson published reports of successful use of Iodine for Graves Hyperthyroidism. (4-6). In the 1930′s and 1940′s, Drs. Thompson, Walcott and Redisch reported on their successful use of Iodine to treat Graves’ Hyperthyroidism. (6-9)
Dr. Redisch in 1940 reported on his case experience which was favorable. One important distinction was made however, between Graves hyperthyroidism and the toxic nodular goiter. Dr Redisch felt that : “Iodine should never be given to patients with old nodular goiters become toxic.”(9)

Left Image” Think Purple, purple tee shirt for Iodine.
The Wisdom of Drs Wolf and Wartofsky
In 1948, the great thyroidologists Wolff and Chaikoff published a report which stated (10):
“we do believe that our findings justify the conclusion that an interference in organic binding of iodine by the gland is an integral part of the mechanism by which iodine brings about a remission in Graves’ disease.” (10)
In 1970, the great thyroidologist, Dr. Wartofsky, published his report on the “Inhibition by iodine of the release of thyroxine from the thyroid glands of patients with thyrotoxicosis.” in the Journal of Clinical Investigation (8). He stated,
“the decreased serum T4 concentration could only have resulted from decreased secretion of the hormone by the gland”,
thus explaining how iodine induces remission in Graves Hyperthyroidism.(11)

Left Image: Microscopic histology slide of thyroid tissue of Graves’ Hyperthyroid patient showing Hyperplasia
thyroid cells lining the the follicular spaces (white areas). This is
caused by overstimulation of the TSH receptor by TSI antibodies.
Iodine Not For Toxic Nodular Goiter
Iodine is contra-indicated in thyrotoxosis caused by toxic nodular goiter. These patients may have a solitary or multiple thyroid nodules, one of which is hyper-functioning. These patients are “toxic”, meaning they are hyperthyroid, because one of these nodules has mutated into a hyper-functioning autonomous nodule.(17-18) In this type of nodule, there has been a mutation in the cell line coding for the TSH receptor causing it to malfunction.. Thus the cells in the autonomous nodule uncontrollably convert iodine into thyroid hormone resulting in hyperthroidism.(17-18)
The Autonomous Thyroid Nodule
My previous article discussed diagnosis and treatment of thyrotoxicosis caused by the autonomous thyroid nodule. The clinical history usually includes some form of Iodine exposure, perhaps obtained from the health food store. Ultrasound thyroid Imaging usually shows a dominant thyroid nodule. Radionuclide imaging wth I-123 or Technetium 99M usually shows the “Hot Nodule” causing the thyrotoxicosis. The toxic nodule may be solitary, or may be present against a background of multiple nodules.

Differentiating Autonomous Nodule from Graves’ Disease in the New Patient Presenting with Thyrotoxicosis
Since Iodine is useful for Graves’ Disease, and is contra-indicated in the autonomous nodule (toxic nodule), it is important to determine whether the thyrotoxicosis is caused by Graves’ Disease or a toxic nodule (Toxic Multinodular Goiter)
Image at upper left shows radioisotope scan with typical appearance of multiple thyroid nodules in Multinodular Goiter courtesy of IJEM.Arrows point to nodules.
Graves’ Disease is an autoimmune thyroid disease in which there are antibodies to the TSH receptor detectable on blood testing. The newer TRAb Thyroid Receptor Antibodies are specific for Graves Disease. They come in two varieties, the stimulatory antibodies called TSI, and the inhibitory antibodies called TBII, (Thyroid binding inhibitory immuneglobulins). Both are sensitive and specific for Graves’ Disease. The newest TBII test is called the H-TBII which uses human material rather than porcine material for the test. (34, 34a)
Dr Wallaschofski from Germany says in a 2004 article, the h-TBII test should be performed on all patients to differentiate Graves’ from Toxic Multinodular Goiter, (34):
“… the h-TBII should be performed in all patients with hyperthyroidism to differentiate Graves’ disease from non-autoimmune hyperthyroidism such as toxic multinodular goitre” quote from (34)

Above
image shows radionuclide thyroid scan. Left Image: normal thyroid
uptake. Right Image: Diffusely enlarged thyroid gland with increased
radiotracer uptake typical appearance of Graves’ Hyperthyroidism.
The thyroid gland in Graves’ disease is usually smoothly enlarged on imaging and palpation (see image above). On the other hand, In the patient with toxic Multi-Nodular Goiter, the thyroid gland is usually irregular and bumpy with either solitary nodule or multiple nodules on imaging.(see the above images).
Modern Treatment of Hyperthyroidism
By 1980, treatment of Graves’ Disease had evolved. New anti-thyroid drugs were developed such as PTU and Methimazole (Tapazole). Thyroid ablation with Radioactive I-131 given as an oral capsule was finding popularity. Of course, surgical thyroidectomy remained a treatment options. (29-33)
However, the original Iodine treatment remains in the hospital formulary for preparation of the Hyperthyroid Patient prior to thyroidectomy.(12-13)
In 2009, Dr Sinem Kiyici reported on the use of Lugol’s Iodine in combination with anti-thyroid drugs for preparation of the hyperthyoid patient for thyroidectomy.(14)

Left Image: typical TED (thyroid eye disease) with retracted eye lids, exopthalmos, and reddened inflamed conjununctiva.
First Line Therapy With Iodine
In 2000, Dr. Jamieson reported on the successful treatment of Graves’ disease in pregnancy with Lugol’s iodine. (15) In 2013, Dr. Gangadharan reported on the use of Iodine as first line therapy in a child with Graves’ Disease.(16)
Lithium and Iodine Combination Treatment (49-56)
Lithium carbonate (300 mg tabs) can be an effective treatment for hyperthyroidism. Dosage is usually 300 mg three times a day. Blood levels are checked to avoid toxicity. (49-56) Lithium inhibits the release of thyroid hormone by the thyroid gland. The mechanism is thought similar to that of Iodine.
Perhaps a more effective treatment for Graves hyperthyroidism is found with the combination of both Lithium Carbonate and Iodine (Lugol’s) used together.(49)
Quoted from the Jonathan Wright MD Newsletter (49):
“In 1972, Dr. R Temple at the Mayo CLinic
published the first clinical investigation of lithium treatment for
Graves’ disease. Using high-dose lithium for 10 individuals, they
reported that thyroid hormone levels fell by 20-30 percent within five
days.
Twenty-six years later, in a review of more than 10 successful trials of lithium therapy for Graves’ disease, the authors wrote: “a small number of studies have documented [lithium's] use in the treatment of patients with Graves’ disease… it’s efficacy and utility as an alternative anti-thyroid [treatment] are not widely recognized…”(55)
They also note lithium’s rapid effect: “Lithium normalizes [thyroid hormone] levels in one to two weeks…” Of course, they also caution that “toxicity precludes its use as a first-line or long-term therapeutic agent.”
But if they’d just added flaxseed oil and vitamin E to their treatment, they would have basically eliminated the risk of toxicity.
In fact, every individual (except one) whom I’ve treated with
iodine-iodide (in the form of Lugol’s Solution) and high dose lithium
has had blood tests for thyroid hormone return to normal within two
weeks. Their tests then stay normal as long as they use the Lugol’s
solution and high dose lithium.” (49-56)
Once such protocol used Lithium Carbonate 300 mg three times a day for three days, then start the Lugol’s Iodine 5 % solution – 3 drops three times a day. (48-56)
The Return of Iodine for Graves’ Disease
Drs Guy Abraham, David Brownstein and George Flechas of the Iodine Project recommend Iodine for treatment of Graves’ Hyperthyroidism. However, They advocate a nutritional supplement program prior to using Iodine: Selenium. Magnesium, Unrefined sea salt and vitamin C. (22-23)
Graves’ is an Autoimmune Disease
Graves’ disease is an autoimmune disease in which auto-antibodies attack and stimulate the TSH receptor. As such, based on the work of Allesio_Fasano, gluten sensitivity and leaky gut has been implicated in the etiology.(48) See my previous article on this.
Underlying gluten sensitivity is the common cause of leaky gut and auto-immune disease. The mechanism of molecular mimicry has been proposed with leakage of bacteria into the blood stream which invokes an immune response. The bacteria, Yersina has been implicated in Graves’ Disease. Antibodies to Yersinia cross react with the TSH receptor, producing hyperthyroidism by stimulating the TSH receptors in the thyroid gland.(36-42). Similarly, thyroid eye disease (TED) is the result of autoimmune attack on TSH receptors or other antigens in the extra-ocular muscles, peri-orbital adipose and connective tissue.(43-47)
Radio-Active Iodine Worsens Thyroid Eye Disease
Over the years, radioactive iodine (I-131) has enjoyed considerable popularity as a treatment for hyperthyroidsm. However, Dr. Jerome M. Hershman laments in 2013 Clinical Thyroidology that radio-Iodine worsens Thyroid Eye Disease (TED).(30)
Dr. Hershmann continues with the following quote: “It is difficult to predict how patients with Graves’ disease will be treated 20 years from now, but I hope that we will have some rational therapy that is directed at the autoimmune origin and that makes our entire current armamentarium obsolete.” (30)
Obviously, there is something yet to be desired with modern treatment of Graves’ Disease. Perhaps treatment with combined Iodine and Lithium is the answer (see below references (49-56).
Addressing the Underlying Auto-Immune Cause
Modern treatment ignores the underlying autoimmune causation, and is concerned solely with controlling the hyperthyroid metabolic state with drugs (methimazole)(31), radioactive iodine and thyroidectomy.(57)
Our program addresses the underlying autoimmune cause.
1) Gluten sensitivity testing with anti-gliadin antibody, and Genetic testing. If positive, a Gluten Free Diet may be curative,
2) Extended Food Reactivity testing, and dietary modification to eliminate reactive foods.
3) Healing the Gut with various nutritional programs including Glutamine, digestive enzymes, probiotics, etc.
4) Selenium level testing and optimization. See my previous article on this.
5) Vitamin D3 level testing and optimization.
6) Low Dose Naltrexone, an immune modulator, has been found useful in autoimmune disease patients.
7) Control and manage hyperthyroid state by inhibiting thyroid function
with Iodine, lithium and methimazole (57). In some cases Beta Blocker
(atenolol, propranolol) may be required to manage tachycardia.
8) Block and Replace: Once thyroid function has been decreased sufficiently with medical treatment (Methimazole, Lithium and/or Iodine), Thyroid Hormone replacement can be started with the goal of suppressing TSH. We prefer to use natural thyroid medication (NDT) such as Naturethroid from RLC labs, rather than T4 only levothyroxine commonly used by endocrinologists and primary carer physicians. By suppressing TSH, we have seen antibodies levels decline in autoimmune thyroid cases.
7) Thyroid Eye Disease (TED) in the Graves’ patient should be managed by coordinated team of physicians, endocrinologists, and ophthalmologists with specialty experience in managing TED. This typically involves a neuro-ophthalmologist, an orbital surgeon, and a strabismus surgeon. Unfortunately the usual treatments with antithyroid drugs and thyroidectomy do not improve thyroid eye disease. In fact, radioiodine therapy for Graves’ disease can worsen thyroid eye disease. (43)

Elaine Moore: A Useful On-Line Resource
Elaine Moore is a Graves’ Disease expert, having gone through it
herself, she has written books covering all aspects of Graves’ Disease,
and is strongly recommended to you. Here is the link to her web site.
Left Image: Book Cover, Graves’ Disease: A Practical Guide by Elaine Moore.

Above Image: Book Cover Advances in Graves’ Disease by Elaine Moore

Left Image: Book Cover Thyroid Eye Disease by Elaine Moore.
Jeffrey Dach MD
7450 Griffin Road Suite 190
Davie Florida 33314
954 792-4663
http://www.bioidenticalhormones101.com/
Articles with related interest:
Thyrotoxicosis from the Autonomous Thyroid Nodule
Hashimotos Thyroiditis, Manic Depression, Psychosis and Psychiatric Manifestations
Iodine and Hashimotos Thyroid Disease
Hashimotos Thyroid Disease and Molecular Mimicry
Hashimotos Thyroiditis and Selenium Part One
Hashimotos, Selenium and Iodine, Part Two
Hashimotos and Iodine Part Three
Selenium and the Thyroid More Good News
——————————————————————————————-
Thanks and Credit goes to Dr. Guy Abraham for most of the information in this article which comes from The Safe and Effective Implementation of Orthoiodosupplementation In Medical Practice by Guy E. Abraham, MD IV. The Use of Inorganic, Non-radioactive Iodine/Iodide in Graves’ Disease.
Also thanks and credit goes to David Brownstein MD for much of the information in this article.
Also thanks to Elaine Moore for her excellent informational webs sites, archives, message boards and books on Graves’ Disease.
Links and References
1) http://www.optimox.com/pics/
The Safe and Effective Implementation of Orthoiodosupplementation In
Medical Practice by Guy E. Abraham, MD IV. The Use of Inorganic,
Non-radioactive Iodine/Iodide in Graves’ Disease
“If this proposed mechanism is valid, orthoiodosupplementation,30 combined with magnesium intake between 800-1,200 mg/day, a daily amount this author recommended 21 years ago 98 for magnesium sufficiency, should reverse autoimmune thyroiditis. This nutritional approach is also effective in Graves’ autoimmune thyroiditis as previously discussed.”
2) http://books.google.com/books?
Lectures on clinical medicine: delivered at the Hôtel-Dieu, Paris, Volume 1 By Armand Trousseau page 586-7
In 1863, Trousseau inadvertently used tincture of iodine successfully in
a patient with exophthalmic goiter.55 “In the course of October, 1863, I
was consulted by a young married lady, who habitually resides in Paris.
She was suffering from subacute exophthalmic goiter… I still found her
heart beat at the rate of 140 to 150 times in the minute… I wished to
administer at the same time tincture of digitalis, but preoccupied with
the idea that there would be some danger in giving iodin, I wrote iodin
instead of digitalis, so that the patient took from 15 to 20 drops of
tincture of iodin a day for a fortnight. (For the reader’s information,
“tincture of iodin” is a 10% solution of iodine in 95% ethanol. The
daily amount ingested was 75-100 mg). When she than came back to me her
pulse was only 90. I found out my mistake, and I substituted tincture of
digitalis for that of iodin, but, after another fortnight, the pulse
had again gone up to 150, so that I at once returned to the iodin.”
Trousseau had the distinction of performing the first double-blind study
of iodine in a cohort of one patient with Graves’ disease. He also
achieved remission of Graves’ disease with prolonged administration of
potassium iodide.56
Thompson, el al,57 in a 1930 publication, quoted several authors in the late 1800s and early 1900s who used Lugol solution alone successfully in Graves’ disease, with complete remission of the disease, eliminating the need for surgery. Destruction of the thyroid gland with goitrogens and radioiodide was fortunately not then available for the management of Graves’ diseased. Professor Kocher came on the scene in the early 1900s and had an adverse iodophobic effect on the treatment of Graves’ disease. Professor Theodore Kocher carried a lot of weight, being the recipient of the Nobel Prize in Medicine and Physiology in 1909, for his work on “thyroid surgery,” the only Nobel Prize assigned to research on the thyroid gland. He was against the use of iodine/iodide in exophthalmic goiter and all forms of hyperthyroidism.58,59
———————–
3) http://www.ncbi.nlm.nih.gov/
Biochem Biophys Res Commun. 1988 Aug 15;154(3):1287-92.
Inhibition by iodide of iodide binding to proteins: the “Wolff-Chaikoff”
effect is caused by inhibition of H2O2 generation. Corvilain B, Van
Sande J, Dumont JE. of iodide oxidation.
——————————
1924
4) http://archinte.jamanetwork.
Starr, Paul, et al. “The effect of iodin in exophthalmic goiter.” Archives of Internal Medicine 34.3 (1924): 355.
1925
5) http://www.nejm.org/doi/pdf/
LAHEY, FRANK H. “The use of iodine in goitre.” The Boston Medical and Surgical Journal 193.11 (1925): 487-490.
1929
6) http://www.jstor.org/stable/25332878
The Treatment Of Exophthalmic Goitre by Charles S. D. Don The
British Medical Journal Vol. 1, No. 3572 (Jun. 22, 1929), pp. 1108-1112
1930
7) http://archinte.jamanetwork.
Thompson WO, Thompson PK, Brailey AG, et al. “Prolonged treatment of
exophthalmic goiter by iodine alone.” Arch Int Med, 1930; 45:481-502.
Orcel, P., et al. “Thompson, WO, Thompson, PK, Brailey, AG, et al, 1930
Prolonged Treatment of Exophthalmic Goiter by Iodine Alone.” Arch. Int.
Med 45: 481-502.
The value of a short intensive course of iodine medication as a
method of preparing a patient with exophthalmic goiter for thyroidectomy
has been unquestioned since the report of Plummer1 in 1923. Following
his announcement, the use of iodine in this disease rapidly became
widespread. It was soon noted by various observers, beginning with
Starr, Segall and Means,2 that in cases in which this medication was
prolonged, its effect, though striking, was usually only temporary; and
that after a few weeks the patient might be as ill as before it was
started, if not worse. Thus, in general, the prolonged treatment for
exophthalmic goiter with iodine alone has come to be regarded as a
futile, if not a dangerous, procedure.
There are, however, some observations in the literature concerning
favorable results in exophthalmic goiter with this method of treatment.
Trousseau,3 in 1863, mentioned a patient in whom the disease was
1934
8) http://archinte.jamanetwork.
Starr, et al 65 from the Massachusetts General Hospital used 15 drops (90 mg) of Lugol gaily for the treatment of exophthalmic goiter, with a 92% success fate, eliminating he need for surgery. “Of these 25 cases, 20 (80%) responded to iodine by a more or less extensive remission of the disease. Of these 20, 12 (48%) responded with the acute iodine resembling the effect produced by subtotal thyroidectomy. In the remaining eight (32%) the remission occurred, but was less extensive. In five unsuccessful cases (20%) two of the patients were pregnant, and one had cardiac decompensation. If these are omitted from the calculation, iodine administration was successful in 20 of 23, or 92% of our hospital cases.”
1940 Redish !!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
9) Redisch_w_Perloff_WH_medical_treatment_hyperthyroidism_Endocrinology_1940_26
http://press.endocrine.org/
Redisch W and Perloff WH. “The medical treatment of hyperthyroidism.” Endocrinology, 1940; 26:221-228.
Treatment with iodine alone is discussed with: a) cures in 10% of the favorable cases, b) no symptoms on iodine in 40%, c) incomplete but definite improvement in 50%.
Iodine should never be given to patients with old nodular goiters become toxic.
1948
10) Iodine_Thyroid_Wolf_Chaikoff_J. Biol. Chem.-1948-Wolff-555-64
Wolff J and Chaikoff IL. “Plasma inorganic iodide as a homeostatic
regulator of thyroid function.” J Biol Chem, 1948; 174:555-564.
Wolff and Chaikoff1 stated: “Ever since the introduction of iodine therapy for the treatment of Graves’ disease by Plummer in 1923,6 the mechanism by which iodine brings about a dramatic remission of signs and symptoms in patients suffering from this disease has attracted considerable attention … we do believe that our findings, even though they deal with normal thyroid tissue, justify the conclusion that an interference in organic binding of iodine by the gland is an integral part of the mechanism by which iodine brings about a remission in Graves’ disease.”
1970
11) http://www.jci.org/articles/
Wartofsky L, Ransil BJ, and Ingbar SH. “Inhibition by iodine of the
release of thyroxine from the thyroid glands of patients with
thyrotoxicosis.” J Clin Invest, 1970; 49:78-86.
Wartofsky, et al5 in 1970 evaluated the effect of Lugol solution, administered at five drops (30 mg iodine/iodide) three times a day in five thyrotoxic patients.
Following a well-designed protocol, they reported, “It is concluded
that the rapid decrease in T4 secretion induced by iodine is not the
result of an acute sustained inhibition of T4 synthesis (the
Wolff-Chaikoff effect), but rather results from an abrupt decrease in the fractional rate of thyroid T4 release.”
“Regardless of whether or not its administration was anteceded and
accompanied by the administration of large doses of methimazole, iodine
induced a rapid decrease in serum T4-127I concentration which could not
be explained by an increase in the peripheral turnover of T4, as judged
from the metabolism of the 131I-labeled hormone. Hence, the decreased
serum T4 concentration could only have resulted from decreased secretion
of the hormone by the gland. ”
1980 Pre-op Iodide in Graves
12) http://www.ncbi.nlm.nih.gov/
N Engl J Med. 1980 Apr 17;302(16):883-5.
Combination of potassium iodide and propranolol in preparation of
patients with Graves’ disease for thyroid surgery. Feek CM, Sawers JS,
Irvine WJ, Beckett GJ, Ratcliffe WA, Toft AD.
Abstract We assessed the efficacy of the combination of propranolol and potassium iodide in the preparation of patients with Graves’ disease for thyroid surgery. Potassium iodide was given orally in a dose of 60 mg three times a day for 10 days before operation in 10 patients who were already receiving propranolol. In contrast to previous experience with either drug used singly, the combined regimen caused a significant fall in mean serum total thyroxine and triiodothyronine to levels in the euthyroid range before operation (P less than 0.001). There was also a significant fall (P less than 0.05) before operation and transient rise after operation in serum reverse triiodothyronine. These preliminary results suggest that the combination of potassium iodide and propranolol may prove to be the optimum preoperative preparation for patients with Graves’ disease.
1988
13) http://www.ncbi.nlm.nih.gov/
Ann R Coll Surg Engl. May 1988; 70(3): 123–127.
Effect of preoperative iodine in patients with Graves’
disease controlled with antithyroid drugs and thyroxine. S. Kaur, J. H.
Parr, I. D. Ramsay, T. M. Hennebry, K. J. Jarvis, and E. Lester
Department of Endocrinology, North Middlesex Hospital, London.
Lugols preop thyroidectomy
2009
14) http://www.endocrine-
Endocrine Abstracts (2009) 20 P67 Rapid preparation of patients with
hyperthyroidism for thyroidectomy. Sinem Kiyici1, Ozen Oz Gul1, Soner
Cander1, Turkay Kirdak2, Oguz Kaan Unal1, Canan Ersoy1, Ercan Tuncel1,
Erdinc Erturk1 & Sazi Imamoglu1
Thyroidectomy is an alternative treatment in the therapy of hyperthyroidism in patients who are non-compliant, drug-resistant or have various side effects to the antithyroid drugs. Preoperative preparation of hyperthyroid patients is extremely important to avoid per operative complications due to severe thyrotoxicosis. We investigated the effects of lugol solution usage with or without thionamides in the rapid preparation of thyroid surgery retrospectively.
Twenty-two patients with Basedow-Graves disease, 19 patients with toxic multinodulary goiter and 3 patients with toxic adenoma were enrolled into the study. Mean ages of patients were 46.6±14.7 years and mean duration of hyperthyroidism was 38.2±59.3 months. The indications of surgical treatment were as follows: unresponsiveness to medical treatment (n=19), pancytopenia (n=9), hepatotoxicity (n=6) allergic reactions (n=3) and noncompliance (n=7) with antithyroid drugs. To restore euthyroidism before surgery, 27 patients treated with lugol solution whereas 17 patients treated with lugol solution and thionamides. Mean dose of lugol solution was daily 27.7±5.5 drops and the mean usage of lugol solution was 9.7±2.3 days. Beta-blocking agents were used in 31 patients. After lugol treatment serum free T4 concentration decreased from 2.5±1.6 to 1.37±0.71 ng/dl (normal range=0.7–1.48 ng/dl) while serum free T3 concentrations decreased from 10.0±7.3 to 3.9±3.7 pg/ml (normal range=1.71–3.71 pg/ml). Percentage changes of serum free T4 and free T3 levels were not different in patients treated with lugol solution alone as compared with patients treated lugol solution and thionamides. All patients were clinically in euthyroid status before surgery. Uneventful total and subtotal thyroidectomy performed in 37 patients while hemithyroidectomy was performed in 7 patients.
In conclusion, lugol treatment with and without antithyroid drugs is safe and effective choice in rapid preparation of patients with hyperthyroidism to thyroidectomy when surgery cannot be delayed.
2000 Pregnancy
15) http://www.ncbi.nlm.nih.gov/
Scott Med J. 2000 Feb;45(1):20-1.
Successful treatment of Graves disease in pregnancy with Lugol’s iodine.
Jamieson A1, Semple CG.
We report a case of Grave’s disease in pregnancy complicated by intolerance of standard antithyroid drug therapy. We describe the success of prolonged use of organic iodine as a primary treatment prior to surgical intervention.
2013 full text pdf
16) Use_of_Iodine_First_Line_Therapy_Graves_Gangadharan_BJMMR_2013
The Use of Iodine as First Line Therapy in Graves’ Disease Complicated
with Neutropenia at First Presentation in a Paediatric Patient
Arundoss Gangadharan1, Harsha Hanumanthaiah1 and Sze May Ng1*
1Department of Paediatrics, Southport and Ormskirk NHS Trust, Wigan
Road, Ormskirk L392AZ, United Kingdom. British Journal of Medicine &
Medical Research, 3(2): 324-328, 2013
———————————————————–
Plummer1924
Plummer HS and Boothby WM. “The Value of iodine in exophthalmic goiter. ” J Iowa Med Soc, 1924; 14:65.
1925
Plummer WA. “Iodin in the ttreatment of goiter.” Med Cl North America, 1925; 8:1145-1151.
——————————
Autonomous Nodule
17) http://www.ncbi.nlm.nih.gov/
Here, we present a case of iodine-induced thyrotoxicosis in a patient with multinodular goiter with autonomously functioning tissue. Initially, the source of iodine was not obvious, especially as iodinated contrast agents, amiodarone, or topical antiseptics were not used. Finally, after intensive questioning it was revealed that the consumption of a kelp-containing tea was the sought-after source of excessive iodine.
18) http://www.ncbi.nlm.nih.gov/
Thyroid. 1998 Jan;8(1):83-100.
Iodine-induced hyperthyroidism: occurrence and epidemiology.
Stanbury JB1, Ermans AE, Bourdoux P, Todd C, Oken E, Tonglet R, Vidor G, Braverman LE, Medeiros-Neto G.
We have critically reviewed the available information on iodine-induced hyperthyroidism (IIH) from published sources and other reports as well as the experience of the authors in Tasmania, Zaire, Zimbabwe, and Brazil. Administration of iodine in almost any chemical form may induce an episode of thyrotoxicosis (IIH). This has been observed in epidemic incidence in several countries when iodine has been given as prophylaxis in a variety of vehicles, but the attack rate as recorded has been low. IIH is most commonly encountered in older persons with long standing nodular goiter and in regions of chronic iodine deficiency, but instances in the young have been recorded. It customarily occurs after an incremental rise in mean iodine intake in the course of programs for the prevention of iodine deficiency, or when iodine-containing drugs such as radiocontrast media or amiodarone are administered. The biological basis for IIH appears most often to be mutational events in thyroid cells that lead to autonomy of function. When the mass of cells with such an event becomes sufficient and iodine supply is increased, the subject may become thyrotoxic. These changes may occur in localized foci within the gland or in the process of nodule formation. IIH may also occur with an increase in iodine intake in those whose hyperthyroidism (Graves’ disease) is not expressed because of iodine deficiency. The risks of IIH are principally to the elderly who may have heart disease, and to those who live in regions where there is limited access to medical care. More information is needed on the long-term health impact of IIH or “subclinical” IIH, especially in the course of prophylaxis programs with iodized salt or iodinated oil in regions where access to health care is limited.
——————————
1975
19) http://press.endocrine.org/
Serum Thyroxine and Triiodothyronine Concentrations During Iodide Treatment of Hyperthyroidism, The Journal of Clinical Endocrinology & Metabolism 40.1 (1975): 33-36. CHARLES H. EMERSON, ARLENE J. ANDERSON, WILLIAM J. HOWARD, and ROBERT D. UTIGER
Serum thyroxine (T4) and triiodothyronine (T3) concentrations were
measured at frequent intervals in 9 hyperthyroid patients treated with
iodide alone. Serum T4 and T3 levels fell initially in all patients. In 6
patients, after a mean fall in serum T4 of 46% and in serum T3 of 47%
after 4 to 11 days of therapy, thyroid hormone levels began to rise. In
the 3 remaining patients a rise in thyroid hormone levels was not seen
following the initial fall. However, in one of this group T4 and T3
levels did not reach the normal range despite 60 days of therapy. These
data support the concept that iodide alone is not an ideal agent for the
treatment of hyperthyroidism.
—————————
2009
20) http://www.ncbi.nlm.nih.gov/
Endocrinology. Mar 2009; 150(3): 1084–1090.
The Sodium-Iodide Symporter NIS and Pendrin in Iodide Homeostasis of the Thyroid Aigerim Bizhanova and Peter Kopp
In vivo data suggest that high concentrations of iodide lead to reduction in both NIS mRNA and protein levels, partially by a transcriptional mechanism. In vitro results suggest that exposure to high doses of iodide results in a decrease in NIS protein levels that is, at least in part, due to an increase in NIS protein turnover
———————
1992
21) http://www.ncbi.nlm.nih.gov/
Clin Endocrinol (Oxf). 1992 Jun;36(6):573-8.
The effect of iodide on serum thyroid hormone levels in normal persons,
in hyperthyroid patients, and in hypothyroid patients on thyroxine
replacement.
Philippou G1, Koutras DA, Piperingos G, Souvatzoglou A, Moulopoulos SD.
To clarify the duration and the extent of the antithyroid effect of
iodides in hyperthyroidism, and to investigate whether iodides have an
additional peripheral effect on the metabolism of thyroid hormones, as
has been reported for some organic iodine compounds.
DESIGN:The effect on the peripheral thyroid hormone levels of 150 mg of
potassium iodide daily (equivalent to 114 mg of iodide) for 3-7 weeks
was compared in 21 hyperthyroid patients and 12 healthy controls. A
possible effect of iodide on the peripheral metabolism of thyroid
hormones was investigated by assessing the serum levels of thyroid
hormone in 12 hypothyroid patients on thyroxine replacement for 2 weeks.
PATIENTS:There were 21 thyrotoxic patients, 12 healthy hospital
controls, and 12 patients with complete or near-complete hypothyroidism,
on thyroxine replacement.
MEASUREMENTS:The following were measured before and at weekly intervals
after iodide administration: (1) pulse rate, (2) serum T4, (3) serum T3,
(4) serum TSH, (5) serum thyroxine-binding capacity (TBC), (6) serum
rT3, (7) serum thyroxine-binding globulin (TBG), (8) the free-T4 Index,
calculated as T4/TBC.
RESULTS:In the hyperthyroid patients serum T4, T3 and rT3 decreased,
whereas serum thyroxine-binding globulin and thyroxine binding capacity
increased. Serum T3, however, did not become completely normal in all
cases. After 21 days, serum T4 and T3 started increasing again in some
cases, but other patients remained euthyroid even after 6 weeks. In the
normal controls there was a small but significant and consistent
decrease in serum T4, T3 and rT3 and an increase in serum TSH. Finally,
in the T4-treated hypothyroid patients there was no consistent change,
except for an increase of serum T4 at 1 and 14 days and a decrease of
serum TSH the first day.
CONCLUSION:Iodides in hyperthyroidism have a variable and unpredictable
intensity and duration of antithyroid effect. Their antithyroid effect
is smaller in normal controls. They have no important effect on the
peripheral metabolism of thyroid hormones.
———————–
22) http://www.optimox.com/pics/
Facts about Iodine and Autoimmune Thyroiditis by Guy E. Abraham, MD
23) http://www.ncbi.nlm.nih.gov/
DEGROOT, LESLIE J., JOAN E. THOMPSON, and ANN D. DUNN. “Studies on an
iodinating enzyme from calf thyroid.” Endocrinology 76.4 (1965): 632-645
—————————
alternating Graves and Hashi
24) http://www.plosmedicine.org/
Alzahrani AS, Aldasouqi S, Abdel Salam S, Sultan A (2005) Autoimmune
Thyroid Disease with Fluctuating Thyroid Function. PLoS Med 2(5): e89.
————–
25) david brownstein interview:
Q. Can those with Hashimoto’s or Graves’ disease take iodine?
A: I explain this topic in much more detail in my book, but let me
summarize the answer. M research has shown that both Hashimoto’s and
Graves’ disease are caused, in part, from low iodine. In fact, nearly
every new patient with either a diagnosis of Hashimoto’s or Graves’
disease has tested significantly low for iodine. My experience has shown
that the vast majority of patients suffering with these illnesses
improve their condition when iodine deficiency is rectified. However,
some people (including those with and without Hashimoto’s and Graves’
disease) may have problems with iodine supplementation. Of course, there
can be an adverse effect to anything, iodine included.
The best results with iodine supplementation occur in those that have
their levels checked and are followed by a health care provider who is
knowledgeable about iodine. Furthermore, iodine supplementation works
better when used as part of a complete holistic treatment regimen.
——————————
26) From GOODMAN & GILMAN’S THE PHARMACOLOGICAL BASIS OF THERAPEUTICS – 11th Ed. (2006) :
Mechanism of Action.
High concentrations of iodide appear to influence almost all important aspects of iodine metabolism by the thyroid gland (Roti and Vagenakis, 2005). The capacity of iodide to limit its own transport has been mentioned above. Acute inhibition of the synthesis of iodotyrosines and iodothyronines by iodide also is well known (the Wolff-Chaikoff effect). This transient, 2-day inhibition is observed only above critical concentrations of intracellular rather than extracellular concentrations of iodide. With time, “escape” from this inhibition is associated with an adaptive decrease in iodide transport and a lowered intracellular iodide concentration, most likely due to a decrease in NIS mRNA and protein (Eng et al., 1999). The mechanism of the acute Wolff-Chaikoff effect remains elusive and has been postulated to be due to the generation of organic iodo-compounds within the thyroid (Pisarev and Gartner, 2000).An important clinical effect of high [I-]plasma is inhibition of the release of thyroid hormone. This action is rapid and efficacious in severe thyrotoxicosis. The effect is exerted directly on the thyroid gland and can be demonstrated in the euthyroid subject as well as in the hyperthyroid patient. Studies in a cultured thyroid cell line suggest that some of the inhibitory effects of iodide on thyrocyte proliferation may be mediated by actions of iodide on crucial regulatory points in the cell cycle (Smerdely et al., 1993).In euthyroid individuals, the administration of doses of iodide from 1.5 to 150 mg daily results in small decreases in plasma thyroxine and triiodothyronine concentrations and small compensatory increases in serum TSH values, with all values remaining in the normal range. However, euthyroid patients with a history of a wide variety of underlying thyroid disorders may develop iodine-induced hypothyroidism when exposed to large amounts of iodine present in many commonly prescribed drugs (Table 56-6), and these patients do not escape from the acute Wolff-Chaikoff effect (Roti et al., 1997). Among the disorders that predispose patients to iodine-induced hypothyroidism are treated Graves’ disease, Hashimoto’s thyroiditis, postpartum lymphocytic thyroiditis, subacute painful thyroiditis, and lobectomy for benign nodules. The most commonly prescribed iodine-containing drugs are certain expectorants, topical antiseptics, and radiological contrast agents.
———-
27) http://rnjournal.com/journal-of-nursing/thryoid-storm-and-the-aacn-synergy-model
nursing case report of thyroid storm treated with Lugols
RNJournal 2014 Journal of Nursing Deborah L. Bray, RN, BSN, CNS Graduate Student Murray State University
Critical care nurses should be familiar with the regimen of medications administered during thyroid storm. The first medication administered should be an anti-thyroid medication such as propylthiouracil (PTU) which blocks the synthesis of thyroid hormones and inhibits the peripheral conversion of T4 to T3. The dosage is 200-250mg every 4 hours orally or via gastric tube. The nurse should monitor for signs of bleeding and a decreased platelet count. Methimazole, another anti-thyroid medication does not work in the periphery as PTU does; therefore, PTU is the anti-thyroid medication of choice during thyroid storm (Dahlen, 2002; Dulak, 2005; Kaplow & Hardin, 2007).
One to two hours later, a potassium iodide solution such as Lugol’s solution should be administered to prevent the release of stored thyroid hormone into the system. Timing of this medication is important as early administration of iodide may cause the body to synthesize more T4, worsening the toxic state.
The dosage is 8 drops every 6 hours orally or via gastric tube
(Dahlen, 2002; Dulak, 2005; Kaplow & Hardin, 2007).(Dahlen, 2002; Dulak, 2005; Kaplow & Hardin, 2007).
———-
preo-op preparation of graves patient using iopanoic acid GB
radiographic contrast, iodineated contrast material instead of iodine
full pdf available
28) Rapid_Preoperative_Prep_Graves_CLAUDIA_PANZER_2004
Rapid Preoperative Preparation for Severe Hyperthyroid Graves’ Disease
CLAUDIA PANZER, ROBERT BEAZLEY, AND LEWIS BRAVERMAN The Journal of Clinical Endocrinology & Metabolism 89(5):2142–2144
2004 . Department of Surgery and Section of Endocrinology, Diabetes, and
Nutrition, Boston Medical Center, Boston,Massachusetts 02118
Conventional preoperative preparation for TX includes antithyroid
drugs and iodine administration before surgery and often takes months to
render patients euthyroid. Far more rapid control of thyrotoxicosis can
be achieved by
the oral administration of iodinated radiographic contrast agents
(IRCAs) such as iopanoic acid (IOP) or ipodate, often given in
combination with corticosteroids and antithyroid drugs, and -blockers.
IRCAs have a multitude of effects on thyroid physiology and thyroid
hormone metabolism. They competitively inhibit types 1 and 2 5
-monodeiodinase in the
liver, brain, and thyroid, thereby blocking the conversion of T4 to T3.
This leads to a rapid and persistent reduction of T3, while reverse T3
levels increase due to decreased clearance of reverse T3 (5– 8). IRCAs
also decrease serum T4 levels inhyperthyroid patients due to a decrease
in the thyroidal organification of iodine and thyroid hormone secretion
from the gland due to the iodine released from these agents (9 –11).
However, serum T4 levels decrease more slowly with IRCAs than with
potassium iodide treatment, probably reflecting the decrease in the
plasma clearance rate of T4 and a decrease in the hepatic uptake of T4
by displacement of T4 from hepatic binding sites (12). Iodine released
from IRCAs also reduces intraoperative blood loss by decreasing thyroid
vascularity
(13–15)
Modern MAnagement
29) Silkiss_Systemic_Mangagement_Graves_Disease_UCSF Systemic
Management of Graves’ Disease Rona Z. Silkiss, M.D., FACS Associate
Clinical Professor, UCSF Chief, Division of Ophthalmic Plastic and
Orbital Surgery
California Pacific Medical Center. PDF slide presentation.
30) Clin_Thyroidol_2013_Survey_Management_Graves_Jerome_Hershman
Clin Thyroidol 2013;25:35–36. A Survey of Management of Uncomplicated Graves’ Disease Shows that Use of Methimazole Is Increasing and Use of Radioactive Iodine Is Decreasing. Jerome M. Hershman, Burch HB, Burman KD, Cooper DS. A 2011 survey of clinical practice patterns in the management of Graves’ disease. J Clin Endocrinol Metab 2012;97:4549-58. Epub October 5, 2012; doi: 10.1210/jc.2012-2802.
The near-uniform avoidance of using RAI in patients with Graves’ ophthalmopathy is striking and attributable mainly to the Italian studies showing that RAI worsens ophthalmopathy and that this can be prevented by corticosteroids (4,5). It is difficult to predict how patients with Graves’ disease will be treated 20 years from now, but I hope that we will have some rational therapy that is directed at the autoimmune origin and that makes our entire current armamentarium obsolete.
———————–
1987 Methimazole
31) http://www.ncbi.nlm.nih.gov/
J Endocrinol Invest. 1987 Jun;10(3):291-5.
Initial treatment of thyrotoxic Graves’ disease with methimazole: a randomized trial comparing different dosages.
Messina M, Milani P, Gentile L, Monaco A, Brossa C, Porta M, Camanni F.
We evaluated the efficacy of different doses of methimazole (MMI) as the
initial therapy for Graves’ disease. Fourteen patients were treated
with 15 mg/die of the drug (group A) and 14 with 30
mg/die (group B). Blood samples for T3, T4, FT3 and FT4 were obtained
before beginning therapy, every 48 h during the first 12 days and on the
45th day of treatment. All these hormonal parameters fell significantly
from the 2nd day of therapy in both groups. All the patients, except
for one in group B, had normal or subnormal levels of thyroidal hormones
on the 45th day of treatment. The comparison between the two groups of
regression coefficients over the first 12 days showed no significant
differences. The absolute decrease of each examined parameter on day 12
was positively correlated with the relevant pretreatment value. These
results demonstrate that doses of MMI (15 mg/die) much lower than those
commonly recommended are able to rapidly control thyroidal
overproduction as effectively as 30 mg/die.
2010 use KI with Methimazole
32) http://www.ncbi.nlm.nih.gov/
Clin Endocrinol (Oxf). 2010 Jun;72(6):845-50. doi: 10.1111/j.1365-2265.2009.
Benefit of short-term iodide supplementation to antithyroid drug treatment of thyrotoxicosis due to Graves’ disease.
Takata K1, Amino N, Kubota S, Sasaki I, Nishihara E, Kudo T, Ito M,
Fukata S, Miyauchi A.Combined treatment with anti-thyroid drugs (ATDs)
and potassium iodide (KI) has been used only for severe thyrotoxicosis
or as a pretreatment before urgent thyroidectomy in patients with
Graves’ disease. We compared methimazole (MMI) treatment with MMI + KI
treatment in terms of rapid normalization of thyroid hormones during the
early phase and examined the later induction of disease remission.
DESIGN AND PATIENTS: A total of 134 untreated patients with Graves’
disease were randomly assigned to one of four regimens: Group 1, MMI 30
mg; Group 2, MMI 30 mg + KI; Group 3, MMI 15 mg and Group 4, MMI 15 mg +
KI. For easy handling, KI tablets were used instead of saturated
solution of KI. KI was discontinued when patients showed normal free
thyroxine (FT4) levels but MMI was continued with a tapering dosage
until remission. Remission rate was examined during a 4- to 5-year
observation.
MEASUREMENTS: Serum FT4, FT3 and TSH were measured by chemiluminescent
immunoassays. TSH receptor antibody (TRAb) was assayed with TRAb-ELISA.
Goitre size was estimated by ultrasonography.
RESULTS: After 2 weeks of treatment, normal FT4 was observed in 29% of
patients in Group 1 and 59% (P < 0.05) of patients in Group 2.
Furthermore, normal FT4 after 2 weeks of treatment was observed in 27%
of patients in Group 3 and 54% (P < 0.05) of patients in Group 4.
Similarly, FT3 normalized more rapidly in Groups 2 and 4 than in Groups 1
and 3. None of the patients showed an increase in thyroid hormones or
aggravation of disease during combined treatment with MMI and KI. The
remission rates in Groups 1, 2, 3 and 4 were 34%, 44%, 33% and 51%,
respectively, and were higher in the groups receiving combined therapy
but differences among four groups did not reach significance.
CONCLUSIONS: Combined treatment with MMI and KI improved the short-term
control of Graves’ hyperthyroidism and was not associated with
worsening hyperthyroidism or induction of thionamide resistance.
33) http://www.ncbi.nlm.nih.gov/
http://www.ncbi.nlm.nih.gov/
Ther Adv Endocrinol Metab. 2011 Jun;2(3):135-44. Current concepts in graves’ disease. Girgis CM1, Champion BL, Wall JR.
Graves’ disease is the most common cause of hyperthyroidism in the
developed world. It is caused by an immune defect in genetically
susceptible individuals in whom the production of unique antibodies
results in thyroid hormone excess and glandular hyperplasia. When
unrecognized, Graves’ disease impacts negatively on quality of life and
poses serious risks of psychosis, tachyarrhythmia and cardiac failure.
Beyond the thyroid, Graves’ disease has diverse soft-tissue effects that
reflect its systemic autoimmune nature. Thyroid eye disease is the most
common of these manifestations and is important to recognise given its risk to vision and potential to deteriorate in response to radioactive iodine ablation. In
this review we discuss the investigation and management of Graves’
disease, the recent controversy regarding the hepatotoxicity of
propylthiouracil and the emergence of novel small-molecule
thyroid-stimulating hormone (TSH) receptor ligands as potential targets
in the treatment of Graves’ disease.
34) http://www.ncbi.nlm.nih.gov/pubmed/15127319
Exp Clin Endocrinol Diabetes. 2004 Apr;112(4):171-4.
TSH-receptor autoantibodies – differentiation of hyperthyroidism between
Graves’ disease and toxic multinodular goitre. Wallaschofski H1, Kuwert
T, Lohmann T.
In conclusion, thyroid-stimulating antibodies in a bioassay or TSH-receptor antibodies detected with the h-TBII assay have the highest diagnostic power to differentiate Graves’ disease from toxic multinodular goitre.
34a)
http://www.ncbi.nlm.nih.gov/pubmed/11589682
Clin Endocrinol (Oxf). 2001 Sep;55(3):381-90.
TSH-receptor antibody measurement for differentiation of hyperthyroidism
into Graves’ disease and multinodular toxic goitre: a comparison of two
competitive binding assays.
Pedersen IB1, Knudsen N, Perrild H, Ovesen L, Laurberg P.
Graves’ disease is characterized by stimulating autoantibodies to the
TSH-receptor (TRAb). The aim of this study was to compare the
performance of a new TRAb assay based on competitive binding to
recombinant human TSH-receptors (H-TRAb) with an assay employing
purified porcine TSH-receptors (P-TRAb). Furthermore, to evaluate the
applicability of the H-TRAb assay to discriminate between patients with
hyperthyroidism due to Graves’ disease (GD) and multinodular toxic
goitre (MNTG).
DESIGN AND MEASUREMENTS: H-TRAb and P-TRAb were measured in patients
with newly diagnosed hyperthyroidism due to GD (n = 106) and MNTG (n =
94). For comparison, TRAb was measured in patients with primary
autoimmune hypothyroidism, euthyroid subjects with an enlarged thyroid
gland by ultrasound, and healthy controls (n = 100 for each group).
Patients were consecutively included from a population survey.
RESULTS: If the cut-off values recommended by the manufacturer for
TSH-receptor antibody positivity were used for evaluation, the
sensitivity of the H-TRAb assay vs. the P-TRAb assay in diagnosing GD
was: 95.3/67.9% (P < 0.001). Specificity was (H/P-TRAb): 99/99%. The
sensitivity of P-TRAb was increased if the upper 97.5% limit of
measurements in controls was used as cut-off (H-TRAb vs. P-TRAb:
95.3/80.2%, P < 0.001). Specificity (H/P-TRAb): 98/98%. The
difference between assay performance may partly be due to a better
technical performance of the H-TRAb assay with more reliable results in
the low range of measurements. However, even in GD patients with clearly
measurable TRAb, 25% had a P-TRAb < 50% of the value expected from
the H-TRAb measurement. This suggests that a subgroup of patients
produce TRAb with a higher affinity for the human than the porcine TSH
receptor. A relatively high proportion of patients with MNTG were TRAb
positive (H-TRAb/P-TRAb: 17/9%). Characteristics of H-TRAb positive and
negative MNTG patients were compared. There was no difference between
size of thyroid gland and number of nodules by ultrasonography. H-TRAb
positive patients had significantly higher serum T4 and T3 and a greater
number were TPO-Ab positive.
CONCLUSIONS: H-TRAb diagnosed Graves’ disease with a high sensitivity
and specificity than P-TRAb. The high occurrence of TRAb in multinodular
toxic goitre might in part reflect an overlap between Graves’ disease
and multinodular toxic goitre in some patients.
35) http://www.ncbi.nlm.nih.gov/
J Endocrinol Invest. 1986 Aug;9(4):287-91.
Effect of sodium ipodate and iodide on free T4 and free T3
concentrations in patients with Graves’ disease. Robuschi G, Manfredi
A, Salvi M, Gardini E, Montermini M, d’Amato L, Borciani E, Negrotti L,
Gnudi A, Roti E.
Graves’ hyperthyroid patients were treated daily for 10 days with 1 g
sodium ipodate, a cholecystographic agent which exerts a blocking effect
on the peripheral conversion of T4 to T3, or with 12 drops of saturated
solution of potassium iodide (SSKI). Serum concentrations of free T4
(FT4) and free T3 (FT3) were measured before, during and 5 and 10 days
after the administration of each drug. Sodium ipodate treatment induced a
rapid decrement of serum FT4 concentrations which declined from 48.9
+/- 6.6 pg/ml to 26.0 +/- 2.7 pg/ml. In these patients serum FT3
concentrations declined from 12.4 +/- 2.0 pg/ml to 2.5 +/- 0.4 pg/ml.
Ten days after sodium ipodate withdrawal, serum FT4 and FT3
concentrations returned to baseline values. In patients treated with
SSKI serum FT4 concentrations declined from 51.1 +/- 8.8 pg/ml to 11.3
+/- 1.4 pg/ml and FT3 from 15.7 +/- 2 pg/ml to 2.6 +/- 0.3 pg/ml.
Moreover, after therapy interruption serum free thyroid hormone
concentrations returned to baseline values in these patients. Serum FT4
pattern during the study was not different between the two groups of
subjects whereas serum FT3 concentrations were significantly lower in
patients treated with sodium ipodate. These findings indicate that SSKI
and sodium ipodate are effective in inducing a rapid decrement of serum
free thyroid hormone concentrations. Therefore the employment of these
drugs may be useful in the treatment of patients with thyroid storm and
those undergoing thyroidectomy
——————————————-
Graves
Graves Hyperthyroidism, Celiac, Gluten, Wheat, Yersinia Abs cross react with TSH Abs, Leaky Gut . Clinical Case Report of 37 y/o female with Graves Hyperthyroidism and Celiac Dx who becomes euthyroid on Gluten Free Diet
36) http://thyroidscience.com/cases/huang.3.09/huang.et.al.3.16.09.pdf
Case Report: Hyperthyroidism, Iron-deficiency Anemia, and Celiac Disease
Cindy Huang, MD, PGY3, Amy 1 Toscano-Zukor, DO,2 Xiangbing Wang, MD,
PhD3 1UMDNJ-Robert Wood Johnson Medical School, Department of Medicine,
One Robert Wood Johnson Place MEB 486, New Brunswick, NJ 08903-0019.
2,3UMDNJ-Robert Wood Johnson Medical School, Department of
Endocrinology, One Robert Wood Johnson Place MEB 384B, New Brunswick, NJ
Abstract.
The objective was to report a case of a woman with celiac disease
presenting with hyperthyroidism and iron-deficiency anemia. Methods. We
report the clinical course of this patient and her laboratory findings.
We highlight the important associations between hyperthyroidism,
iron-deficiency anemia, and celiac disease. The literature is reviewed
for the typical and atypical presentations of celiac disease in relation
to hyperthyroidism
and iron-deficiency anemia. Results. A 37-year-old woman presented with
symptoms of hyperthyroidism and was found to have iron-deficiency
anemia. During the work up for iron-deficiency anemia, she was diagnosed
with celiac disease on small-bowel biopsy. After being placed on a
gluten free diet, symptoms of hyperthyroidism improved without
anti-thyroid medication.
Conclusion. Our case demonstrates that routine screening for celiac disease should be highly considered for patients with both hyperthyroidism and
Graves and Yersinia
1986
37) http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1542330/
Clin Exp Immunol. 1986 May;64(2):249-54.
Thyrotrophin (TSH) binding sites on Yersinia enterocolitica recognized by immunoglobulins from humans with Graves’ disease. Heyma P, Harrison LC, Robins-Browne R. Abstract
Antibodies against the gram negative enteric bacterium Yersinia enterocolitica have been found in a high proportion of persons with autoimmune thyroid disorders, especially in those with Graves’ disease or hyperthyroidism (Shenkman & Bottone, 1981). There is strong evidence that Graves’ disease is caused by receptor autoantibodies which mimic the bioeffects of thyroid stimulating hormone (TSH) on the thyroid (Manley, Knight & Adams, 1982).
Recently, saturable binding sites for TSH were demonstrated in Y. enterocolitica under non-physiological conditions (Weiss et al., 1983). We have characterized TSH binding sites on Y. enterocolitica under physiological conditions and studied their interaction with Graves’ immunoglobulins (Ig’s). Saturable and specific binding of receptor-purified 125I-TSH to lysozyme/EDTA-treated Y. enterocolitica (serotype 03) was demonstrated under both non-physiological and physiological conditions. Scatchard binding plots were linear indicating a single class of binding site (Kd 1 X 10(-7) M, maximum of 30,000 binding sites per cell). In the presence of Graves’ Ig’s the binding of 125I-TSH to Y. enterocolitica was significantly inhibited. Graves’ Ig’s also precipitated a protein of relative molecular mass (Mr) 64,000 from Triton-solubilized, 125I-labelled Y. enterocolitica, similar in size to one of the proteins precipitated by Graves’ Ig’s from human thyroid membranes.
These findings are consistent with the hypothesis that thyroid autoimmunity may be triggered by bacterial infection via a mechanism involving crossreactivity at the level of the TSH receptor. They also suggest that elements of mammalian endocrine systems are highly conserved and have a function in prokaryotes.
1987
38) http://www.ncbi.nlm.nih.gov/pubmed/3618088
Acta Med Austriaca. 1987;14(1):11-4.
[Antibodies to Yersinia enterocolitica in immunogenic thyroid diseases].
[Article in German] Petru G, Stünzner D, Lind P, Eber O, Möse JR.
In 1976 Shenkman et al. revealed that in patients with thyroid disorders antibodies against Yersinia enterocolitica could be demonstrated in increased frequency. In 1983 Ingbar et al. first established that the gram-negative bacterium Yersinia enterocolitica shows on its surface saturable binding sites for thyrotropin (TSH). If such binding sites resemble immunologically human TSH receptors this would indicate that TSH receptor antibodies could be produced in selected individuals having been infected with bacteria showing TSH receptors. The aim of our study was to compare the incidence of antibodies against Yersinia enterocolitica in two groups of thyroid disorders which are either immunogenic (Graves’ disease and Hashimoto thyroiditis) or non-immunogenic (toxic adenomas, endemic goitre). In our series of 111 patients antibodies against Yersinia enterocolitica were demonstrated in a significantly higher percentage (36.3%) in patients suffering from immunogenic than in patients with non-immunogenic thyroid disorders (19.6%). The antibody titres were mainly directed towards Yersinia subtypes 8 and 3. It may, therefore, be assumed that the gram-negative bacterium Yersinia enterocolitica may have an active part in triggering immunogenic thyroid diseases such as Graves’ disease or Hashimoto thyroiditis.
1990
39) http://www.ncbi.nlm.nih.gov/pubmed/2083529
Endocrinol Jpn. 1990 Aug;37(4):489-500. Antibodies to Yersinia
enterocolitica serotype 3 in autoimmune thyroid diseases. Takuno H,
Sakata S, Miura K. Third Department of Internal Medicine, Gifu
University School of Medicine, Japan.
Abstract The prevalence of increased titres of antibodies to Yersinia enterocolitica (serotype 3) has been studied in sera from patients with various thyroid diseases. In contrast to the low prevalences of the antibodies in healty subject (24.3%), titres (greater than 10) of anti-Yersinia enterocolitica (anti-Yersinia) were found more frequently in patients with thyroidal disorders, especially in Graves’ disease (70.0%).
Furthermore, high titres of the antibodies (greater than or equal to 160) were found only in patients with Graves’ disease.
There was no significant correlation between the titers of anti -Yersinia antibodies and those of anti-TSH receptor antibodies in sera from patients with Graves’ disease. In seven individual samples of sera, the anti- Yersinia antibody titer was high before treatment, but the decrease in the anti-TSH receptor antibody titer following treatment was associated with a simultaneous decline in anti-Yersinia antibodies in all of them. A highly positive and significant correlation between the titers of anti-TSH receptor antibodies and anti-Yersinia antibodies was obtained in each of them. These findings could be merely a reflection of the measurement of the cross-reaction of anti-Yersinia antibodies with anti-TSH receptor antibodies but the possibility of an association between Yersinia infection and the production of anti-TSH receptor antibodies in at least some patients with Graves’ disease cannot be ruled out.
2002
40) http://www.ncbi.nlm.nih.gov/pubmed/12193307
Thyroid. 2002 Jul;12(7):613-7. Relationship between thyroid
autoimmunity and Yersinia enterocolitica antibodies. Corapçioglu D,
Tonyukuk V, Kiyan M, Yilmaz AE, Emral R, Kamel N, Erdogan G. Source
Department of Endocrinology and Metabolic Diseases, Ankara University
School of Medicine, Ankara, Turkey.
It has previously been proposed that subclinical Yersinia enterocolitica infection may play a role in autoimmune thyroid disease (AITD). In this study, we investigated the relationship between the thyroid autoantibodies and the antibodies that produced against different serotypes of Y. enterocolitica. A total of 215 subjects were included into the study (65 newly diagnosed Graves’ disease [GD], 57 Hashimoto’s thyroiditis [HT], 53 nontoxic diffuse goiter [NTDG], and 40 subjects for control group [CG]). Thyroid receptor antibodies (TRAb), thyroid and agglutinating antibodies against Y. enterocolitica serotype O:3, O:5, O:8, O:9 were measured in the blood samples. The highest incidence of Y. enterocolitica antibody positivity was measured in GD (53.8% for O:3, 29.2% for O:5, 44.6% for O:8, and 40% for O:9) and followed by HT. In patients with GD, TRAb levels were also higher than in patients with HT, NTDG, and CG. There was no difference between NTDG and CG in respect to the titer levels and the positivity of both TRAb and Y. enterocolitica antibodies. There was also a weak linear correlation between TRAb level and the titer of antibodies against Y. enterocolitica antigens. It can be concluded that Y. enterocolitica infection may play a role in etiology of GD in Turkey.
2008
41) http://www.ncbi.nlm.nih.gov/pubmed/18284638
Clin Endocrinol (Oxf). 2008 Sep;69(3):491-6. Epub 2008 Feb 18.
Too early to dismiss Yersinia enterocolitica infection in the aetiology
of Graves’ disease: evidence from a twin case-control study. Brix TH,
Hansen PS, Hegedüs L, Wenzel BE. Department of Endocrinology and
Metabolism, Odense University Hospital, Odense, Denmark.
Yersinia enterocolitica (YE) infection has long been implicated in the pathogenesis of Graves’ disease (GD). The association between YE and GD could, however, also be due to common genetic or environmental factors affecting the development of both YE infection and GD. This potential confounding can be minimized by investigation of twin pairs discordant for GD. We first conducted a classical case-control study of individuals with (61) and without (122) GD, and then a case-control study of twin pairs (36) discordant for GD. Immunoglobulin (Ig)A and IgG antibodies to virulence-associated Yersinia outer membrane proteins (YOPs) were measured. The prevalence of YOP IgA and IgG antibodies.
RESULTS: Subjects with GD had a higher prevalence of YOP IgA (49% vs. 34%, P = 0.054) and YPO IgG (51% vs. 35%, P = 0.043) than the external controls. The frequency of chronic YE infection, reflected by the presence of both IgA and IgG YOP antibodies, was also higher among cases than controls (49%vs. 33%, P = 0.042). Similar results were found in twin pairs discordant for GD. In the case-control analysis, individuals with GD had an increased odds ratio (OR) of YE infection: IgA 1.84 (95% CI 0.99-3.45) and IgG 1.90 (95% CI 1.02-3.55). In the co-twin analysis, the twin with GD also had an increased OR of YE infection: IgA 5.5 (95% CI 1.21-24.81) and IgG 5.0 (95% CI 1.10-22.81).
CONCLUSION: The finding of an association between GD and YE in the case-control study and within twin pairs discordant for GD supports the notion that YE infection plays an aetiological role in the occurrence of GD, or vice versa.
2011
42) http://online.liebertpub.com/doi/abs/10.1089/thy.2010.0364
http://www.ncbi.nlm.nih.gov/pubmed/21877932
Thyroid. 2011 Nov;21(11):1283-4. Epub 2011 Aug 30.
Bioinformatics support the possible triggering of autoimmune thyroid
diseases by Yersinia enterocolitica outer membrane proteins homologous
to the human thyrotropin receptor. Guarneri F, Carlotta D, Saraceno G,
Trimarchi F, Benvenga S.
_______________________________
thyroid eye disease
43) http://www.ncbi.nlm.nih.gov/
Clin Ophthalmol. 2009; 3: 543–551. Published online Oct 19, 2009.
Update on thyroid eye disease and management Erick D Bothun, Department
of Ophthalmology, University of Minnesota, MMC 493, 420 Delaware Street
Erick D Bothun, Ryan A Scheurer, [...], and Michael S Leehyroid eye
disease includes infectious and inflamamatory orbital conditions such as
orbital myositis, idiopathic orbital inflammatory syndrome, and orbital
cellulitis. Rarer conditions include orbital neoplasms and carotid
artery – cavernous sinus fistulas. New onset diplopia may result from
cranial nerve palsies, internuclear ophthalmoplegia, or myasthenia
gravis.
Management Patients with Graves’ ophthalmopathy should be managed by a
coordinated team of primary care physicians, endocrinologists, and
ophthalmologists with specialty experience in managing TED. This
typically involves a neuro-ophthalmologist, an orbital surgeon, and a
strabismus surgeon. The complicated nature of treatment often requires
coordination of medical, surgical and radiation therapy.22 Uncontrolled
thyroid function is associated with more severe thyroid eye
disease.23,24 However, antithyroid drugs and surgical
subtotal/near-total thyroidectomy therapies typically do not improve the
ophthalmic disease course.25,26 In fact, radioiodine therapy for
Graves’ disease can exacerbate ophthalmic disease;
44) http://www.
Medical management of thyroid eye disease Dawn D. Yang, MD Mithra
O. Gonzalez, MD, Vikram D. Durairaj, MD,FACSemail address
University of Colorado Denver School of Medicine, Department of
Ophthalmology, Division of Oculofacial Plastic and Reconstructive
Surgery, United States. Saudi Journal of Ophthalmology Volume 25, Issue
1 , Pages 3-13, January 2011
The thyroid stimulating hormone receptor (TSHR) is over-expressed in orbital fibroblasts and adipose tissue in TED patients
Higher levels of TSHR mRNA expression are found in patients with
clinically active disease when compared to patients with inactive TED
Methimazole, carbimazole, and propylthiouracil are the main drug
treatments blocking thyroid hormone synthesis. They are frequently used
in an attempt to achieve remission or as preparative therapy before
radioactive iodine or thyroid surgery. After active concentration by the
thyroid, the drugs inhibit thyroid peroxidase mediated iodination of
tyrosine residues in thyroglobulin. Propylthiouracil had the additional
benefit of inhibiting the peripheral conversion of thyroxine (T4) into
triiodothyronine (T3).
Characteristic enlargement of the extraocular muscles and proliferation
of adipocytes result in the clinical findings, such as eyelid
retraction, exophthalmos, and strabismus of TED.
Conclusion Thyroid eye disease is a common cause of orbital disease in
adults. The pathogenesis is an active area of research and many
immunologic aspects of the disease have been elucidated. Research of
serum markers may assist in the diagnosis, prognosis and response to
therapy through their ability to improve the classification of TED
patients. Characteristic enlargement of the extraocular muscles and
proliferation of adipocytes result in the clinical findings, such as
eyelid retraction, exophthalmos, and strabismus of TED. All patients
require management of their systemic thyroid disease. Most cases of TED
can be managed conservatively. Symptomatic treatment with lubrication
often suffices for those with mild TED. Individuals with moderate to
severe TED may require an escalation of therapy including steroids,
radiation or immunomodulation. Intravenous glucocorticoids have the best
response rate and the lowest incidence of side effects and thus play a
significant role in the management of moderate to severe as well as
sight-threatening TED. Surgical management is required when medical
management fails. An emerging understanding of the immunologic
pathogenesis will likely evolve the management of TED over the next
decade.
45) http://www.ncbi.nlm.nih.gov/
Br J Ophthalmol. Jun 2005; 89(6): 724–729. Quantification of cells
expressing the thyrotropin receptor in extraocular muscles in thyroid
associated orbitopathy
A Boschi, Ch Daumerie, [...], and M C Many
46) http://www.ncbi.nlm.nih.gov/
CMAJ. Aug 1, 2006; 175(3): 239. Autoimmunity against eye-muscle
antigens may explain thyroid-associated ophthalmopathy. Junichi Tani
and Jack R. Wall
autoimmunity against a thyroid-stimulating hormone receptor
(TSH-r)–like protein in the orbital preadipocyte and, possibly,
extraocular muscle fibre.
autoimmunity against TSH-r, expressed in fat and connective tissue,
could explain the development of pretibial myxedema, acropachy and the
OCT component of TAO
the eye-muscle and OCT–fat reactions are both autoimmune disorders that
can occur alone or together in patients with thyroid autoimmunity,
manifesting as 3 subtypes of TAO: ocular myopathy, congestive
ophthalmopathy or mixed disease. In ocular myopathy, double vision,
reduced eye movement and increased volumes of eye muscle (seen with
orbital imaging techniques) result from damage to the eye muscles. In
congestive ophthalmopathy, eye swelling, redness, chemosis and increased
tearing are caused by inflammation in the periorbital tissues. Mixed
disease is the most common manifestation of TAO. Chronic eyelid lag,
occurring alone or with other features of TAO, may be a fourth subtype
full pdf
47) Pathogenesis_thyroid-associated_ophthalmopathy_Clinical_Ophthal_2010
Pathogenesis of thyroid-associated ophthalmopathy: does autoimmunity
against calsequestrin and collagen XIII play a role? Clinical
Ophthalmology 2010:4 417–425. Hooshang Lahooti Kishan R Parmar Jack R
Wall The Department of Medicine, University of Sydney, Nepean Clinical
School, Penrith, NSW,
Australia
A popular theory is that the primary reaction may involve antibodies targeting
the TSH-r in the OCT which leads to orbital inflammation,
manifest as orbital fibroblast stimulation, collagen
and glycosaminoglycans (GAGS) production and associated
congestive eye features
—————
ful pdf
48) Zonulin_Regulation_Intestinal_Barrier_Physiol_Rev_2011_Allesio_Fasano
Fasano, Alessio. Zonulin and Its Regulation of Intestinal Barrier
Function: The Biological Door to Inflammation, Autoimmunity, and Cancer.
Physiol Rev 91: 151–175, 2011;
——————————–
49) http://wrightnewsletter.com/2011/09/08/reversing-hyperthyroidism/
Reversing hyperthyroidism by Jonathan Wright MD
I have used that treatment for my patients with tremendous success ever since that study was released. In fact, every individual (except one) whom I’ve treated with iodine-iodide (in the form of Lugol’s Solution) and high dose lithium has had blood tests for thyroid hormone return to normal within two weeks. Their tests then stay normal as long as they use the Lugol’s solution and high dose lithium.
I have my patients use five drops of Lugol’s iodine three times a day for two or three days. Then I have them add 300 milligrams of lithium carbonate three times a day in addition to the Lugol’s. Lugol’s Solution is available at any pharmacy, but you’ll have to get a prescription from your physician.
50) http://www.eje.org/content/94/2/174.short
Lithium and iodine combination therapy for thyrotoxicosis. Acta endocrinologica, 94(2), 174-183. Boehm, T. M., Burman, K. D., Barnes, S., & Wartofsky, L. (1980). Walter Reed Army Hospital
In order to compare the relative therapeutic efficacy of iodine (I) and lithium (Li) in thyrotoxicosis, investigate possible additive effects of these agents, and examine their effect upon thyroidal release, 17 thyrotoxic patients were assigned to groups given either I (n = 9) or Li (n = 8) alone during an initial treatment period, with the alternate drug added as combination treatment during a second treatment period. Half of the patients received methimazole (MMI). Three additional thyrotoxic patients received I during both treatment periods to evaluate the possibility of cumulative I effect upon thyroidal release during the second treatment period. A double isotope technique was utilized as an index of thyroidal release, employing 125I as an intra-thyroidal label and [131I] T4 as a marker of T4 disposal. During the first treatment period either I or Li induced comparable, significant (P < 0.05) decreases in thyroidal release, as measured by slopes of ratios of serum PB125I: PB131I and by percentage inhibition of fractional T4 release rate. In response to Li, there was a 55% decrease in the slope of PB125I: PB131I with MMI and a 52% decrease without MMI.
In response to I, there was a 70% decrease in the slope of PB125I: PB131I with MMI and a 57% decrease without MMI.
Further significant (P < 0.05) decreases in the slopes of these ratios during the second, combined treatment period (I + Li) occurred only in those patients who had initially received I. No further decreases in the second treatment period were seen in patients receiving I during both treatment periods. Thus, I and Li together display additive inhibition of thyroidal release only if I is administered initially, but the combination, if Li is used first, does not appear to be more effective than Li alone.
51) http://www.ncbi.nlm.nih.gov/pubmed/989049
J Clin Endocrinol Metab. 1976 Sep;43(3):606-13.
Sensitivity to lithium in treated Graves’ disease: effects on serum T4, T3 and reverse T3.
Burman KD, Dimond RC, Earll JM, Wright FD, Wartofsky L.
Department of Endocrinology and Metabolism, Walter Reed Army Medical Center Washington, D.C. 20012
Seven patients judged to be euthyroid following treatment of diffuse toxic goiter were studied to determine if they were susceptible to lithium induced hypothyroidism. Lithium carbonate was administered for 4-7 weeks in a dosage (900 mg/day) which maintained serum lithium levels between 0.5-1.0 mEq/l. Blood was obtained weekly for the determination of serum 3,5,3′-triiodothyronine (T3), thyroxine (T4), 3,3′,5′-TRIIODO-L-thyronine (reverse T3, rT3) and thyrotropin (TSH). Values observed during lithium therapy were compared to those obtained prior to, and approximately one week after discontinuing lithium.
During the pretreatment preiod, mean (+/- SE) serum T3, T4, and rT3 concentrations were
130 +/- 21 ng/100 ml, 7.6 +/- 0.4 mug/100 ml and 48 +/- 8 ng/100
ml, respectively, and decreased during lithium administration with the
lowest T3, T4 and reverse T3 concentrations of the lowest T3, T4 and
reverse T3 concentrations of
92 +/- 8 ng/100 ml, 4.9 +/- 0.6mug/100 ml, and 33 +/- 6 ng/100, ml, respectively, being reached between the fourth and sixth weeks of study. Thereafter, and in spite of continued treatment with lithium, values for serum concentrations of T3, T4, and rT3 plateaued, or actually increased in 4, 6, and 5 subjects, respectively. Serum TSH concentrations remained 3.0 muU/ml or less throughout the study in 6 patients; 2 of these subjects had no TSH response to thyrotropin-releasing hormone (TRH), even though they had been euthyroid for 3 and 10 months. These data suggest that patients euthyroid following treatment of diffuse toxic goiter display sensitivity to the antithyroid effects of lithium. Furthermore, these observations support the thesis that the inhibitory effects of lithium and iodine upon thyroid hormone synthesis or secretion may involve a similar mechanism of action since increased thyroidal iodine content may be a consequence of therapy with either agent.
52) http://www.ncbi.nlm.nih.gov/pubmed/6423365
Dtsch Z Verdau Stoffwechselkr. 1984;44(1):26-31.
[Results of lithium treatment in severe hyperthyroidism].
[Article in German] Wünsch C, Heberling HJ. Abstract
Up to the present time lithium therapy is under discussion in patients with severe, particularly of contrast remedy induced hyperthyroidism. The aim of our presentation was to investigate if the short term combined methimazole-lithium therapy in the initial phase will be advantageous in contrast to the monotherapy of methimazole. The examination in our material showed a good effectiveness and tolerance of the combined therapy. At present the precise mechanism of action of lithium has not been elucidated. There is evidence that lithium inhibits the thyreoglobulin hydrolysis and the peripheral conversion of thyroxin to triiodothyronin. Other actions are under discussion. In our opinion only the combined methimazole-lithium therapy will be advantageous. Through this procedure an earlier drug effect could be expected and the increase of thyroid hormones after finishing lithium therapy will be suppressed. In the own material severe side effects are not demonstrable.
Lithium as Treatment for Thyrotoxicosis
similarity of inhibition of iodine release from the thyroid produced by Li(+) and iodides
53) http://www.ncbi.nlm.nih.gov/
J Clin Invest. 1972 Oct;51(10):2746-56.
The use of lithium in the treatment of thyrotoxicosis.
Temple R, Berman M, Robbins J, Wolff J.
Since lithium has been shown to inhibit release of iodine from the thyroid, we have investigated its therapeutic potential in thyrotoxicosis. Eight detailed (131)I kinetic studies were performed on seven thyrotoxic women and data was analyzed using a computer program. Lithium at serum levels of about 1 mEq liter decreased the loss of (131)I from the thyroid, led to a fall in serum (131)I levels and diminished urinary (131)I excretion. Computer simulation of the lithium effect required, in every case, that lithium inhibit hormonal and nonhormonal thyroid iodine release. In five cases a second lithium effect was required for a satisfactory fit of the model solution with observed data: namely, an inhibition of hormone disappearance from serum. NEITHER INHIBITION OF RELEASE NOR OF HORMONE DISAPPEARANCE SEEMED TO BE AFFECTED BY METHIMAZOLE (RELEASE: 52% decrease without methimazole, 60% with methimazole; hormone disappearance: approximately 60% decrease in both). When Li(+) was discontinued, recovery of the iodine release rate and hormone disappearance rate over the observed time span was variable, ranging from no recovery to rates that exceeded pre-Li(+) values. When Li(+) is used alone its effect on serum hormone levels is diminished due to continued accumulation of iodide by the thyroid. Thus, serum thyroxine-iodine levels fell 21-30% in 6-8 days in patients who did not receive methimazole and 15-67% in the methimazole-treated subjects. For prolonged therapy, therefore, a thiocarbamide drug must be used in conjunction with Li(+). The similarity of inhibition of iodine release from the thyroid produced by Li(+) and iodides is discussed.
54) http://www.ncbi.nlm.nih.gov/
Lancet. 1976 Mar 20;1(7960):603-5.
Lithium carbonate in the treatment of thyrotoxicosis. A controlled trial.
Kristensen O, Andersen HH, Pallisgaard G.
Of 24 patients with newly diagnosed thyrotoxicosis, 13 were randomly selected for treatment with methimazole 40 mg per day, and 11 for treatment with lithium carbonate in such doses that the serum lithium lay between 0-5 and 1-3 meq. per litre. The lithium treatment brought about a fall in serum-thyroxine iodine (T4I) of 27.0%, and in the free-thyroxine index (F.T.I.) of 38.1% after 10 days. A comparison of the two patient groups with regard to the fall in F.T.I. after 3 and 10 days showed no statistically significant difference; similarly the calculated confidence limits appeared to exclude any difference of clinical importance. 8 of the 11 patients subjected to lithium treatment had side-effects, so that the general condition, which was already affected by the hyperthyroidism, was worsened. It is concluded that lithium cannot be considered superior to thiocarbamides for the rapid control of thyrotoxicosis.
The Endocrinologist 1998; 8: 383-387 Case Report: PDF Only. The Use of Lithium in the Treatment of Hyperthyroidism.
Benbassat, Carlos A. M.D.; Molitch, Mark E. M.D.
We describe four patients treated with lithium for the control of
hyperthyroidism. Conventional therapy with propylthiouracil and/or
methimazole was tried initially, but the patients were either
unresponsive or developed side effects during the drug administration. Within several days of reaching therapeutic levels of lithium, a euthyroid state was achieved in three of the four cases.
Our observations support the use of lithium as an alternative
antithyroid drug in the treatment of hyperthyroidism in certain defined
indications, a clinical use that is not widely known.
56) http://www.ncbi.nlm.nih.gov/pubmed/16912350
Hong Kong Med J. 2006 Aug;12(4):254-9.
full pdf file: Lithium in Thyrotoxicosis Hong Kong 2006
Use of lithium in the treatment of thyrotoxicosis.
Ng YW1, Tiu SC, Choi KL, Chan FK, Choi CH, Kong PS, Ng CM, Shek CC.
To evaluate the efficacy and safety of lithium in the treatment of
thyrotoxicosis, and to study the dose and serum levels at which
therapeutic response occurs.
Thyroid clinic of a regional hospital in Hong Kong.
PATIENTS:Thirteen patients with thyrotoxicosis pending
therapy with radioiodine or surgery, in whom thionamides were
contra-indicated due to adverse reactions or failure of treatment.
MAIN OUTCOME MEASURES:Free thyroxine levels, time to euthyroidism, and side-effects of lithium.
RESULTS:A satisfactory response, defined as a fall by 40% or
more in free thyroxine levels and clinical improvement, was achieved in
eight patients within 1 to 2 weeks of lithium therapy. In four others,
response occurred in 3 to 5 weeks. Response was slow and inadequate in one patient due to ‘escape’. The median dosage of lithium was 750 mg daily, with a range of 500 to 1500 mg daily. The median serum lithium level was 0.63 mmol/L. Lithium toxicity was observed in one patient.
CONCLUSIONS:A relatively low dose of lithium offers a safe and effective
alternative means of controlling thyrotoxicosis in patients who cannot
tolerate or do not respond to thionamides.
————————–
57) Fumarola-et-al.-2010-Medical-Treatment-of-Hyperthyroidism-State-of-the Art
Fumarola, A., Di Fiore, A., Dainelli, M., Grani, G., & Calvanese,
A. (2010). Medical treatment of hyperthyroidism: state of the art.
Experimental and clinical endocrinology & diabetes, 118(10), 678.
58) Lithium Pretreatment in I131 Therapy for hyperthyroidism Płazińska, Maria Teresa, Leszek Królicki, and Marianna Bąk. “Lithium carbonate pre-treatment in 131-I therapy of hyperthyroidism.” Nuclear Medicine Review 14.1 (2011): 3-8.
—————————————————————-
http://www.ncbi.nlm.nih.gov/pubmed/7204887
J Endocrinol Invest. 1980 Oct-Dec;3(4):419-24.
Iodine treatment of iodine-induced thyrotoxicosis.
Boehm TM, McLain J, Burman KD, deShazo R, Wartofsky L.
Abstract
A 62-year-old female who had received prolonged iodine therapy for asthma presented with severe thyrotoxicosis and severe asthma. Her history, elevated serum thyroxine and triiodothyronine, low 131I uptake, and elevated intrathyroidal iodine content by fluorescent scan were most consistent wiht a diagnosis of iodine-induced thyrotoxicosis (IITT). The clinical course of her thyrotoxicosis was protracted, and in spite of its etiologic role in the precipitaton of thyrotoxicosos, iodine was therapeutically efficacious, although combined treatment with methimazole was required to ultimately restore euthyroidism. Therapy with lithium was also employed but appeared to be only transiently effective and combined no additional decrement in serum T4 than that seen with iodine alone. The case exemplifies the heterogeneity of what is considered “iodine-induced” thyrotoxicosis, the complexities inherent in establishing a diagnosis of IITT, and the use of other rapid acting pharmacologic agents in IITT when beta blockade is contraindicated by asthma.
images
http://commons.wikimedia.org/wiki/File:Hyperthyroidism_%282%29.jpg
Histopathological image of diffuse hyperplasia of the thyroid gland clinically presenting as hyperthyroidism. Another view of the same case shown in a filename “Hyperthyroidism_(1).jpg”.H & E stain.
http://upload.wikimedia.org/wikipedia/commons/8/8f/Proptosis_and_lid_retraction_from_Graves%27_Disease.jpg
Photograph showing a classic finding of Graves’ Disease, proptosis and lid retraction.
Date 15 August 2011 Source The Eyes Have It Author
Jonathan Trobe, M.D. – University of Michigan Kellogg Eye Center
http://medcell.med.yale.edu/histology/endocrine_systems_lab/graves_disease.php
Grave’s Disease
Graves’ Disease is a type of hyperthyroidism that is due to the
production of autoantibodies against the TSH receptor on the follicular
epithelial cells. These antibodies mimic the effects of TSH and cause
overproduction and release of thyroid hormone. The epithelial cells in
this patient no longer surround round follicles of colloid. Instead,
these follicles are disappearing and the colloid is becoming depleted.
Compare to normal thyroid.
http://medcell.med.yale.edu/histology/endocrine_systems_lab/images/graves_disease.jpg
Figure 3 Above: Radioactive iodine thyroid scan. Comparison between scans from the normal patient and a patient with Graves’ disease. Note the overall increased uptake throughout the enlarged thyroid gland in a patient with Graves’ disease.
http://www.learningradiology.com/archives05/COW%20154-Thyroid%20Ophthalmopathy/thyroidophcorrect.htm
Cat scan orbital muscle hypertrophy Graves Disease
http://www.physio-pedia.com/images/2/2c/Wiki_image_1.jpg
Graves Hyperthyrodiism
link to this article:
http://wp.me/p3gFbV-1lm
Jeffrey Dach MD
7450 Griffin Road, Suite 190
Davie, Fl 33314
954-792-4663
www.jeffreydach.com
www.drdach.com
www.naturalmedicine101.com
www.bioidenticalhormones101.com
www.truemedmd.com
Click Here for: Dr Dach’s Online Store for Pure Encapsulations Supplements
Click Here for: Dr Dach’s Online Store for Nature’s Sunshine Supplements
Web Site and Discussion Board Links:
jdach1.typepad.com/blog/
disc.yourwebapps.com/Indices/244124.html
disc.yourwebapps.com/Indices/244066.html
disc.yourwebapps.com/Indices/244067.html
disc.yourwebapps.com/Indices/244161.html
disc.yourwebapps.com/Indices/244163.html
Disclaimer click here: www.drdach.com/wst_page20.html
The reader is advised to discuss the comments on these pages with his/her personal physicians and to only act upon the advice of his/her personal physician. Also note that concerning an answer which appears as an electronically posted question, I am NOT creating a physician — patient relationship. Although identities will remain confidential as much as possible, as I can not control the media, I can not take responsibility for any breaches of confidentiality that may occur.
Copyright (c) 2014 Jeffrey Dach MD All Rights Reserved. This article may be reproduced on the internet without permission, provided there is a link to this page and proper credit is given.
FAIR USE NOTICE: This site contains copyrighted material the use of
which has not always been specifically authorized by the copyright
owner. We are making such material available in our efforts to advance
understanding of issues of significance. We believe this constitutes a
‘fair use’ of any such copyrighted material as provided for in section
107 of the US Copyright Law. In accordance with Title 17 U.S.C. Section
107, the material on this site is distributed without profit to those
who have expressed a prior interest in receiving the included
information for research and educational purposes.

